“NOT YOUR GRANDFATHER'S HDM2”
Oncolyze is developing what we hope to be a transformational approach to cancer treatment. It all starts with a molecule called HDM2 (human double minute-binding protein-2). This is a protein that regulates cell growth. In fact, it counterbalances another protein called p53, which is a ‘tumor suppressor’. Most cancers overexpress HDM2 inside the cell, thereby blocking the p53 tumor suppressive function.
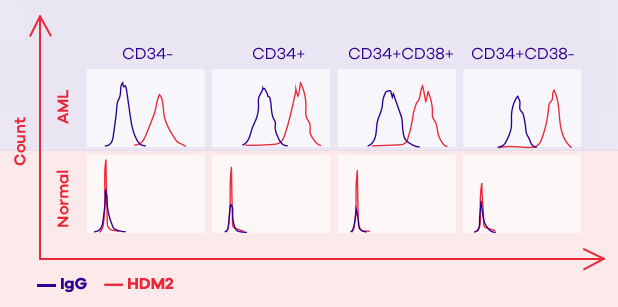
However, researchers at SUNY Downstate Medical Center (from where Oncolyze has licensed the technology) discovered that cancer cells have a high concentration of HDM2 not only intracellularly, but also on the external surface of their cell membrane. Whether this is used to somehow communicate with other cells, we are not yet certain, but what we do know is that more than 80% of cancer types we have tested have a high amount of HDM2 present on their cell surface, whereas normal cells appear to hardly have any. The unique expression of extracellular HDM2 on cancer cells makes it an excellent, selective target for an anti-cancer therapeutic agent.
Our therapeutic approach does exactly this. We target the extracellular HDM2 for a therapeutic mechanism that works at the cell surface and does not rely on the intracellular HDM2 and its function on p53. We know this because our drug candidates work on p53-null cell lines (cancer cells that lack p53 protein).